The restor3d difference
What Sets us Apart
Our innovative approach to personalized musculoskeletal care is inspired by a passion for advancing the field and a commitment to providing greater value to patients, surgeons, and hospitals. We leverage the latest technology and research to create products that enable surgeons to repair the human body in new and effective ways.
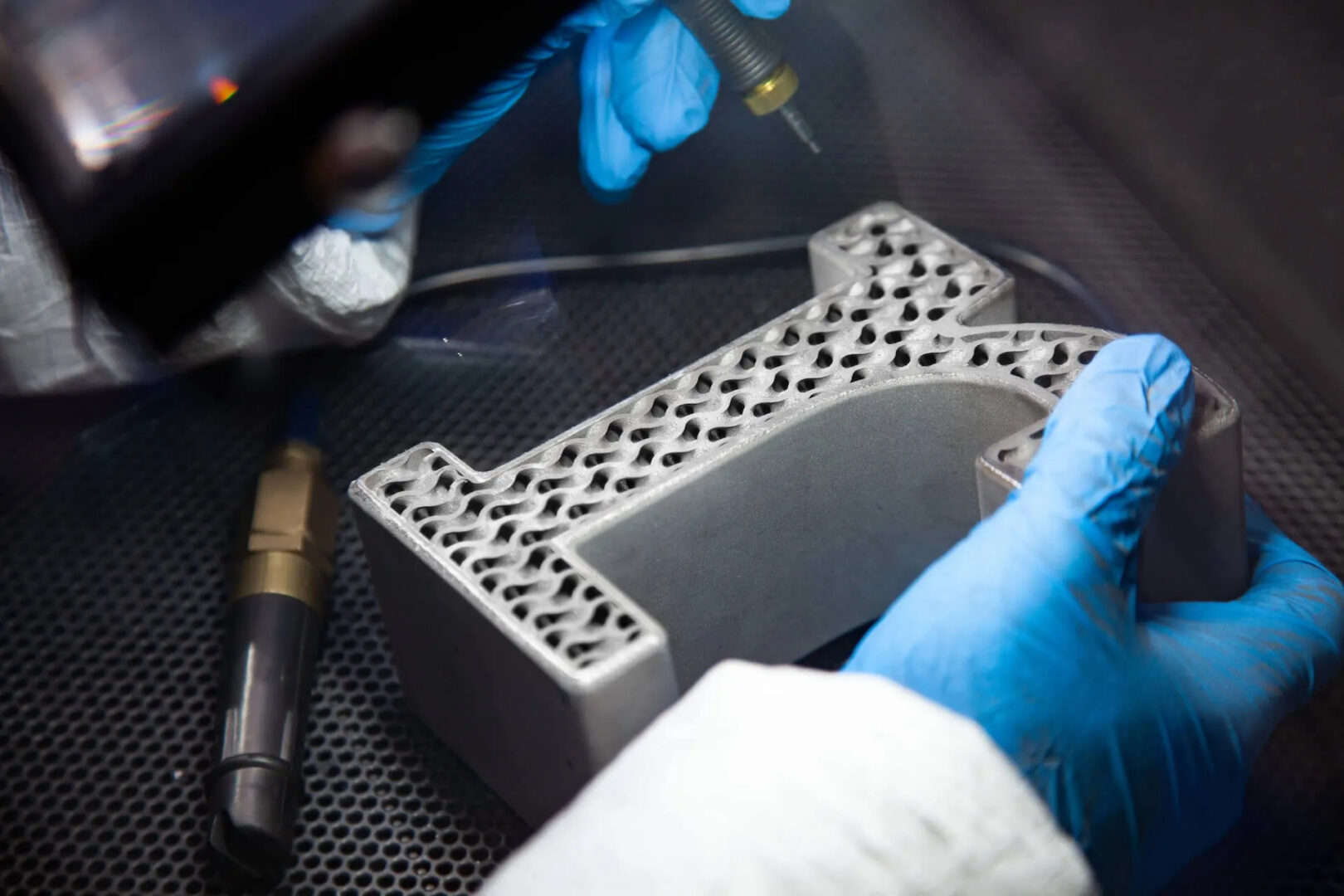
Advanced Additive Manufacturing
We have a deep understanding of the latest additive manufacturing methods. We also stay up to date on device-specific mechanical and biological performance. Our knowledge is based on the scientific research we sponsor at Duke University.
In-House Design and Manufacturing
At restor3d, we utilize a vertically integrated workflow. All of our design work is completed in-house with the assistance of design automation, as well as most of our manufacturing.
Industry-Leading Speed
We’re proud to manufacture safe and effective medical devices at industry-leading speed. We know speed and quality are critical to patient care and successful outcomes. That’s why we strive to deliver our products as efficiently as possible.
We Value
Our Manufacturing Process

Leadership
Our leadership is dedicated to innovation and excellence, making orthopedic treatment accessible and effective for all.
Board of Directors
Our Board of Directors is behind us every step of the way as we continue to push technology forward.
Careers
A Company Where You’ll Make a Difference
Join a team where your work can make a global impact. At restor3d, our additive manufacturing jobs directly benefit patients and surgeons worldwide. We prioritize patient health and provide effective solutions tailored to surgeons’ needs, delivering industry-leading value to our clients.
Contact Us
Get in Touch
How can we help you? Fill out the form and our team will be happy to help.